battery
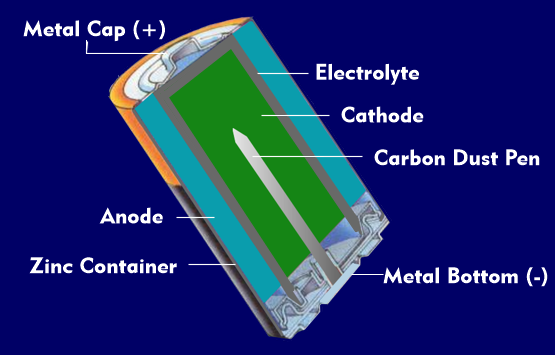
A battery consists of several battery cells. A battery cell is a non-rechargeable energy storage device based on electrochemical conversion. It is called a primary cell, in contrast to rechargeable batteries, which are called secondary cells. A battery cell consists of two electrodes, the anode and the cathode, which are made of different materials and are in an electrolyte.
Such an element, in which two different materials are in one electrolyte and in which DC voltage is generated by electrochemical conversion, is called a galvanic element or galvanic battery cell. The anode of a galvanic battery cell is negatively charged, the cathode positively charged.
Electrochemical reactions take place at the interfaces between the two electrodes and the electrolyte, which can be measured as a voltage value between the electrodes. The voltage value depends on the electrode material and the electrochemical reaction and is called the electrochemical voltage series. Depending on the materials, voltage values between +2.9 V and -3.0 V can occur.
Most batteries use zinc (Zn), carbon or manganese (Mn). Usually the negative electrode, the anode, is made of zinc and is in the form of a container into which the electrolyte and positive electrode are placed. Battery cells usually have a source voltage of 1.5 V. The positive electrode, the carbon electrode, often contains electrolytically produced manganese oxide in addition to manganese dioxide. Common batteries on the market are zinc-carbon batteries, zinc-air batteries, alkaline-manganese batteries( ZnMnO2), zinc-mercury oxide batteries, and lithium batteries. A few percent mercury (Hg) is added to the zinc electrodes to improve performance. Since the zinc cup partially dissolves during discharge, it is surrounded by a steel shell.
Parameters and characteristic values of batteries
Important parameters of batteries are the nominal voltage or open-circuit voltage, the battery capacity, given in ampere-hours( Ah), the internal resistance, the nominal load and the energy density as the ratio of capacity to weight.
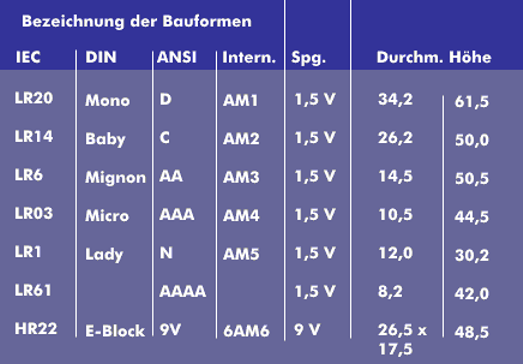
In terms of design, a distinction is made between round batteries, which are all 1.5 V, flat batteries (4R61), which consist of three round batteries and are 4.5 V, 9 V block batteries (6R62) and button cells.
The environmental impact of batteries is described in the Battery Ordinance(BattV) in which the limits for pollutants and disposal are regulated. One of the most environmentally friendly batteries is the paper battery. It does not use chemical elements harmful to the environment and can be composted.